Your automotive battery is one of the most important, and most often overlooked, electrical components in your vehicle. If your battery is not in good condition, you can be stranded, and other components in your vehicle may be damaged. This article discusses how batteries work and how to take care of them.
Your automotive battery is one of the most important, and most often overlooked, electrical components in your vehicle. If your battery is not in good condition, you can be stranded, and other components in your vehicle may be damaged. This article discusses how batteries work and how to take care of them.
What is Inside an Automotive Battery?
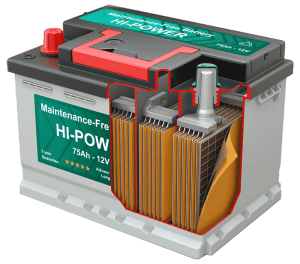 Inside a typical car battery are six smaller energy-producing components called cells. Each cell contains a series of electrodes or plates. The positive plate of the battery is lead [eroxide (PbO2). The negative plates of the battery are pure lead in a soft, sponge-like state. The plates within each cell are arranged in alternating layers for a total of 16 components. All of the positive plates in a cell are wired in parallel, as are all of the negative plates.
Inside a typical car battery are six smaller energy-producing components called cells. Each cell contains a series of electrodes or plates. The positive plate of the battery is lead [eroxide (PbO2). The negative plates of the battery are pure lead in a soft, sponge-like state. The plates within each cell are arranged in alternating layers for a total of 16 components. All of the positive plates in a cell are wired in parallel, as are all of the negative plates.
Each cell produces roughly 2 volts of electricity. The six individual cells are wired in series with one another so the voltage generated by each cell adds together. The result is 12 volts.
Are You Ready for the Chemistry?
A diluted solution of sulfuric acid (H2SO4) surrounds the plates. The ratio of acid to water (H2O) is typically in the region of three parts of water to one part of acid.
When we connect a load to the external terminals of the battery, a chemical reaction starts to take place. Our diluted sulfuric acid mixture comprises H2SO4 and water (H20). As the reaction commences, the sulfuric acid splits into positive hydrogen ions (2H) and negative sulfate Ions (SO4).
 When the hydrogen ions reach the lead peroxide plate, they absorb electronics from it and become a hydrogen atom. This process attacks the lead peroxide to produce lead oxide (PbO) and water (H2O). The lead oxide reacts with the sulfuric acid to form lead sulfate (Pb SO4) and water (H2O).
When the hydrogen ions reach the lead peroxide plate, they absorb electronics from it and become a hydrogen atom. This process attacks the lead peroxide to produce lead oxide (PbO) and water (H2O). The lead oxide reacts with the sulfuric acid to form lead sulfate (Pb SO4) and water (H2O).
Negative sulfate ions move freely within the solution. As they reach the pure lead plate, they give up their extra electron and become what is known as a radical sulfate. As a radical sulfate cannot exist on its own, it will attack the pure lead plate to produce lead sulfate (PbSO4).
The action of positive hydrogen ions taking electrons from the lead peroxide plate, and the negative sulfate ions giving electrons to the pure lead plate produce an electron imbalance. These electrons flow through the external load to try and balance themselves. This process is how the battery provides power to our load (light, amplifier, heater or computer).
The Chemistry behind Battery Charging
 When we apply an external DC source to the battery, we reverse the process. An external DC source such as an alternator or a battery charger feeds electrons to our positive lead sulfate-covered lead peroxide plate and the negative lead peroxide-covered lead plate. During the charging process, the density of the sulfuric acid solution falls, but we still have positive hydrogen ions and negative sulfate ions.
When we apply an external DC source to the battery, we reverse the process. An external DC source such as an alternator or a battery charger feeds electrons to our positive lead sulfate-covered lead peroxide plate and the negative lead peroxide-covered lead plate. During the charging process, the density of the sulfuric acid solution falls, but we still have positive hydrogen ions and negative sulfate ions.
The positively charged hydrogen Ions more toward the negative terminal of the external DC source. Each hydrogen ion takes one electron from the negative plate to become a hydrogen atom. These hydrogen atoms attack the lead sulfate to produce lead and sulfuric acid.
The negative sulfate ions move toward the positively charged plate. When they get there, they give up their extra electron to become radical sulfates. This radical sulfate reacts with the lead sulfate, and forms lead peroxide and sulfuric acid.
We Can Simplify that a Lot!
In a nutshell, the negative terminal of a lead-acid battery has an over-abundance of electrons. When you connect a load to the battery, the electrons scramble through the load to get to the positive terminal. This electron flow is what allows the battery to provide energy to do work.
When we apply a voltage to the battery that is higher than its resting voltage the electron flow reverses. The sulfate layers on the plates are converted back to lead and sulfuric acid.
Battery Charging: Calm Down – What’s the Rush?
About the worst thing you can do to a car battery is to rush the charging process. If you rush the recharging chemical reaction, the lead sulfate will heat up and adhere permanently to the lead and lead peroxide plates. Once it is stuck there, we can no longer use that area of the plate to flow electrons, and we have reduced the effective size of the battery.
 You probably have heard the expression “a battery is never the same after it has been killed.” This statement is very true if the battery is not charged gently and thoroughly.
You probably have heard the expression “a battery is never the same after it has been killed.” This statement is very true if the battery is not charged gently and thoroughly.
When you want to recharge your battery properly, keeping the process slow will allow the chemical reaction to take place at a controlled rate. If you are using a high-quality, computer-controlled charger (and you should be!) there are two major charging stages. The first stage is called bulk charging. The charger will maintain a constant current flow to the battery by adjusting the applied voltage.
How do you know if you are charging a care battery too quickly? Standard flooded batteries should not exceed roughly 120 degrees Fahrenheit during charging. We suggest that slower and cooler is always better. An absorbed glass mat (AGM) or gel battery should not exceed 100 degrees.
Once approximately 80% of the used energy has been returned to the battery, the charger will switch to the absorption stage. At that stage, the charger provides a constant voltage to the battery and the current flow diminishes as the battery reaches full charge.
How to Calculate Maximum Battery Charging Rates
A relatively large car battery may have a capacity of 70 or 80 amp-hours. This specification means that under ideal conditions, you can draw 1 amp of current from the battery for 70 or 80 hours. After that time, the battery will be considered dead.
To find the ideal charging rate for our 70 amp-hour battery, we divide this specification by 10 to get seven amps. The battery should be able to accept 7 amps of charging current without overheating. It is worth noting that, if the battery is completely discharged, it will take 10 hours to charge it. Remember, slower is better when it comes to charging batteries.
Taking Care of Your Car Battery

Some of us who are more fanatical about the care and maintenance of our car batteries will connect them to intelligent battery chargers several times a year. One rule of thumb is to charge your battery fully after each oil change, or four times a year. You should increase this frequency if you make short trips that do not provide adequate charging time. Likewise, time spent playing your audio system with the engine off can drain a battery very quickly. If you have been out with friends and your car battery has been depleted, put it on a high-quality charger overnight.
If you can access the acid solution in your battery, ensure that it is at the proper level, or at the very least, is covering the lead plates completely. A hydrometer should be used to confirm the specific gravity of the solution, but if it is low, adding distilled water is better than doing nothing. That little green “eye” included in some batteries is a hydrometer. When it disappears, the chemical balance within the battery is off and it needs to be charged.
Your local mobile electronics retailer may have a battery load tester that they use before every remote car starter they install. If you are concerned about the condition of your battery, ask them to check it. Being stranded due to a dead battery when the temperatures get cold is frustrating if you are trying to get home or to work.
Ensure the battery terminals and connections to your vehicle are clean and secure at all times. A loose connection can have a dramatic adverse effect on the functionality of your electrical system.
If you need a new battery, check with your local mobile enhancement retailer first. They often have extensive experience in upgrading batteries and can help you choose a solution that will ensure your car is ready to go every time you turn the key.
This article is written and produced by the team at www.BestCarAudio.com. Reproduction or use of any kind is prohibited without the express written permission of 1sixty8 media.